Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 C absorber material on melt progression and chemical forms of iodine or cesium under severe accident conditions
C absorber material on melt progression and chemical forms of iodine or cesium under severe accident conditionsHidaka, Akihide
Insights Concerning the Fukushima Daiichi Nuclear Accident, Vol.4; Endeavors by Scientists, p.341 - 356, 2021/10
Gou llo, M.*; Hokkinen, M.*; Suzuki, Eriko; Horiguchi, Naoki; Barrachin, M.*; Cousin, F.*
llo, M.*; Hokkinen, M.*; Suzuki, Eriko; Horiguchi, Naoki; Barrachin, M.*; Cousin, F.*
Progress in Nuclear Energy, 138, p.103818_1 - 103818_10, 2021/08
Times Cited Count:4 Percentile:56.94(Nuclear Science & Technology)The present work aimed to study the transport of caesium iodide particles through a Thermal Gradient Tube (TGT) from 1023 K to 453 K. Retention inside the tube was evaluated for laminar flowrates composed of argon and steam. Higher retention of particles was highlighted for the experiments using higher steam content and lower flowrate. The second phase of the experiment aimed at identifying the possible revaporization or/and resuspension processes after the deposition. Three atmosphere compositions (Ar/H O, Ar/H
O, Ar/H and Ar/Air) were investigated. The particles removed from what was deposited on the surface walls during the sampling phase exhibited a similar GMD in Ar/H
and Ar/Air) were investigated. The particles removed from what was deposited on the surface walls during the sampling phase exhibited a similar GMD in Ar/H O and Ar/H
O and Ar/H and a bigger diameter in Ar/Air. The experimental results were then analysed with the SOPHAEROS module of the ASTEC code. Overall, the results obtained during the first phase were in agreement with the measured experimental results and during second phase led to no resuspension process.
and a bigger diameter in Ar/Air. The experimental results were then analysed with the SOPHAEROS module of the ASTEC code. Overall, the results obtained during the first phase were in agreement with the measured experimental results and during second phase led to no resuspension process.
Miyahara, Naoya; Miwa, Shuhei; Gou llo, M.*; Imoto, Jumpei; Horiguchi, Naoki; Sato, Isamu*; Osaka, Masahiko
llo, M.*; Imoto, Jumpei; Horiguchi, Naoki; Sato, Isamu*; Osaka, Masahiko
Journal of Nuclear Science and Technology, 57(12), p.1287 - 1296, 2020/12
Times Cited Count:5 Percentile:53.85(Nuclear Science & Technology)In order to clarify the cesium iodide (CsI) transport behavior with a focus on the mechanisms of gaseous iodine formation in the reactor coolant system of LWR under a severe accident condition, a reproductive experiment of CsI transport behavior was conducted using a facility equipped with a thermal gradient tube. Various analyses on deposits and airborne materials during transportation could elucidate two mechanisms for the gaseous iodine formation. One was the gaseous phase chemical reaction in Cs-I-O-H system at relatively high-temperature region, which led to gaseous iodine transport to the lower temperature region without any further changes in gas species due to the kinetics limitation effects. The other one was the chemical reactions related to condensed phase of CsI, namely those of CsI deposits on walls with surface of stainless steel to form Cs CrO
CrO compound and CsI aerosol particles with steam, which were newly found in this study.
compound and CsI aerosol particles with steam, which were newly found in this study.
Miwa, Kazuji; Obata, Hajime*; Suzuki, Takashi
Journal of Nuclear Science and Technology, 57(5), p.537 - 545, 2020/05
Times Cited Count:2 Percentile:21.58(Nuclear Science & Technology)This study investigated the vertical distribution of Iodine-129 ( I) which is mainly produced by European nuclear reprocessing plants in the Chukchi Sea and Bering Sea.
I) which is mainly produced by European nuclear reprocessing plants in the Chukchi Sea and Bering Sea.  I was found to be distributed almost uniformly in fallout level, and an increasing in
I was found to be distributed almost uniformly in fallout level, and an increasing in  I concentration levels caused by high
I concentration levels caused by high  I water inflow from the Atlantic Ocean was not observed. Additionally, we revealed the vertical distribution of iodide, one chemical form of iodine, from the Bering Shelf area to the Chukchi Sea for the first time. The increasing tendency of iodide near sea bottom was observed.
I water inflow from the Atlantic Ocean was not observed. Additionally, we revealed the vertical distribution of iodide, one chemical form of iodine, from the Bering Shelf area to the Chukchi Sea for the first time. The increasing tendency of iodide near sea bottom was observed.
 as a source of atmospheric I
as a source of atmospheric I
Watanabe, Kosuke*; Matsuda, Shohei; Cuevas, C. A.*; Saiz-Lopez, A.*; Yabushita, Akihiro*; Nakano, Yukio*
ACS Earth and Space Chemistry (Internet), 3(4), p.669 - 679, 2019/04
Times Cited Count:8 Percentile:43.59(Chemistry, Multidisciplinary)The photooxidation of aqueous iodide ions (I
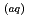 ) at sea surface results in the emission of gaseous iodine molecules (I
) at sea surface results in the emission of gaseous iodine molecules (I
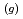 ) into the atmosphere. It plays a certain role in the transport of iodine from ocean to the atmosphere in the natural cycle of iodine. In this study, we determined the photooxidation parameters, the molar absorption coefficient (
) into the atmosphere. It plays a certain role in the transport of iodine from ocean to the atmosphere in the natural cycle of iodine. In this study, we determined the photooxidation parameters, the molar absorption coefficient (
 (
( )) and the photooxidative quantum yields (
)) and the photooxidative quantum yields (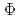
 (
( )) of I
)) of I
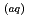 , in the range of 290-500 nm. Through the investigation of the influence of pH and dissolved oxygen (DO) on
, in the range of 290-500 nm. Through the investigation of the influence of pH and dissolved oxygen (DO) on 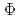
 (
( ), the subsequent emission rates of I
), the subsequent emission rates of I
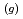 following the photooxidation of I
following the photooxidation of I
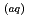 in deionized water solution (pH 5.6, DO 7.8 mg L
in deionized water solution (pH 5.6, DO 7.8 mg L ) and artificial seawater solution (pH 8.0, DO 7.0 mg L
) and artificial seawater solution (pH 8.0, DO 7.0 mg L ) were estimated. A global chemistry-climate model employed herein to assess the I
) were estimated. A global chemistry-climate model employed herein to assess the I
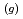 ocean emission on a global scale indicated that the photooxidation of I
ocean emission on a global scale indicated that the photooxidation of I
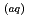 by solar light can enhance the atmospheric iodine budget by up to
by solar light can enhance the atmospheric iodine budget by up to  8% over some oceanic regions.
8% over some oceanic regions.
 C absorber material on melt progression and chemical forms of iodine or cesium under severe accident conditions
C absorber material on melt progression and chemical forms of iodine or cesium under severe accident conditionsHidaka, Akihide
Nihon Genshiryoku Gakkai Wabun Rombunshi, 14(1), p.51 - 61, 2015/03
B C used mainly for BWR and EPR absorbers could cause phenomena which never happen in PWR with Ag-In-Cd absorbers during severe accident. B
C used mainly for BWR and EPR absorbers could cause phenomena which never happen in PWR with Ag-In-Cd absorbers during severe accident. B C would make a eutectic interaction with stainless steel and enhance melt relocation. Boron oxidation could increase H
C would make a eutectic interaction with stainless steel and enhance melt relocation. Boron oxidation could increase H generation and change of liberated carbon to CH
generation and change of liberated carbon to CH could enhance CH
could enhance CH I generation. HBO
I generation. HBO generated during B
generated during B C oxidation could be changed to CsBO
C oxidation could be changed to CsBO by combining with Cs. This may increase Cs deposition in reactor coolant system. There could be differences in configuration, surface area, stainless steel-B
by combining with Cs. This may increase Cs deposition in reactor coolant system. There could be differences in configuration, surface area, stainless steel-B C weight ratio between B
C weight ratio between B C powder and pellet absorbers. Present issue is to clarify effect of these differences on full scale melt progression, B
C powder and pellet absorbers. Present issue is to clarify effect of these differences on full scale melt progression, B C oxidation and source term. Advancement of this research domain could contribute to further sophistication of prediction tool for melt progression and source terms, and treatment of organic iodide formation in safety evaluation.
C oxidation and source term. Advancement of this research domain could contribute to further sophistication of prediction tool for melt progression and source terms, and treatment of organic iodide formation in safety evaluation.
 I
IShirasu, Yoshiro; Minato, Kazuo
Journal of Nuclear Materials, 320(1-2), p.25 - 30, 2003/09
Times Cited Count:4 Percentile:31.64(Materials Science, Multidisciplinary)no abstracts in English
Mineo, Hideaki; Goto, Minoru; Iizuka, Masaru*; Fujisaki, Susumu; Uchiyama, Gunzo
Journal of Nuclear Science and Technology, 39(3), p.241 - 247, 2002/03
Times Cited Count:26 Percentile:82.31(Nuclear Science & Technology)no abstracts in English
Maruyama, Yu; Shibazaki, Hiroaki*; Igarashi, Minoru*; Maeda, Akio; Harada, Yuhei; Hidaka, Akihide; Sugimoto, Jun; Hashimoto, Kazuichiro*; Nakamura, Naohiko*
Journal of Nuclear Science and Technology, 36(5), p.433 - 442, 1999/05
Times Cited Count:9 Percentile:57.31(Nuclear Science & Technology)no abstracts in English
Onuki, Kaoru; Ioka, Ikuo; Futakawa, Masatoshi; Nakajima, Hayato; Shimizu, Saburo; *
Zairyo To Kankyo, 46(2), p.113 - 117, 1997/00
no abstracts in English
Maruyama, Yu; Igarashi, Minoru*; Hashimoto, Kazuichiro; Nakamura, Naohiko*; Hidaka, Akihide; Sugimoto, Jun
Transactions of the American Nuclear Society, 75, p.273 - 274, 1996/00
no abstracts in English
Hidaka, Akihide; Igarashi, Minoru*; Hashimoto, Kazuichiro; ; *; Sugimoto, Jun
Journal of Nuclear Science and Technology, 32(10), p.1047 - 1053, 1995/10
Times Cited Count:14 Percentile:77.92(Nuclear Science & Technology)no abstracts in English
*; ; *; *
Kozan Chishitsu, 40(5), p.323 - 336, 1990/00
no abstracts in English
; Shimizu, Saburo; Nakajima, Hayato; Ikezoe, Yasumasa;
Int.J.Hydrogen Energy, 12(8), p.555 - 559, 1987/08
Times Cited Count:4 Percentile:58.05(Chemistry, Physical)no abstracts in English
Minato, Kazuo; ;
JAERI-M 87-024, 18 Pages, 1987/02
no abstracts in English
Saeki, Masakatsu
AERE-R-11974, p.317 - 332, 1986/00
no abstracts in English
; ; ; ; *
Hoken Butsuri, 21, p.9 - 15, 1986/00
no abstracts in English
;
Hoken Butsuri, 20(2), p.109 - 119, 1985/00
no abstracts in English
 XY
XY crystals; Rb
crystals; Rb ZnI
ZnI ,Tl
,Tl ZnI
ZnI ,and Cs
,and Cs HgI
HgI
Japanese Journal of Applied Physics, 24(SUPPL.24-2), p.387 - 389, 1985/00
Times Cited Count:109 Percentile:96.26(Physics, Applied)no abstracts in English
Nakajima, Hayato; Shimizu, Saburo; ; Ikezoe, Yasumasa;
Nihon Kagakkai-Shi, 8, p.1257 - 1261, 1984/00
no abstracts in English